Views: 0 Author: Site Editor Publish Time: 2025-06-19 Origin: Site








The preform mold industry plays a pivotal role in the manufacturing of plastic bottles and containers used across various sectors. Two significant segments within this industry are medical and cosmetic preform molds. Understanding the differences between Medical Preform Mold and Cosmetic Preform Mold is essential for manufacturers aiming to meet industry standards and regulatory requirements. This article delves into the distinct characteristics, applications, and regulatory considerations of medical and cosmetic preform molds.
Material selection is a critical factor that differentiates medical preform molds from cosmetic ones. Medical preform molds often require materials that comply with stringent medical-grade standards. These materials must be biocompatible, sterile, and free from contaminants. Commonly used materials include high-grade polyethylene terephthalate (PET) and medical-grade polypropylene.
In contrast, cosmetic preform molds prioritize aesthetic appeal and versatility. While they also use PET and other plastics, the focus is on clarity, color options, and the ability to incorporate design elements. Additives may be used to enhance visual appeal, which is generally acceptable in the cosmetic industry but may not meet medical industry standards.
Medical preform molds demand high precision and tight tolerances to ensure the safety and efficacy of medical products. The molds must produce components that fit seamlessly with other medical devices or packaging, preventing leaks and contamination. Advanced technologies like computer-aided design (CAD) and computer-aided manufacturing (CAM) are extensively used to achieve the required precision.
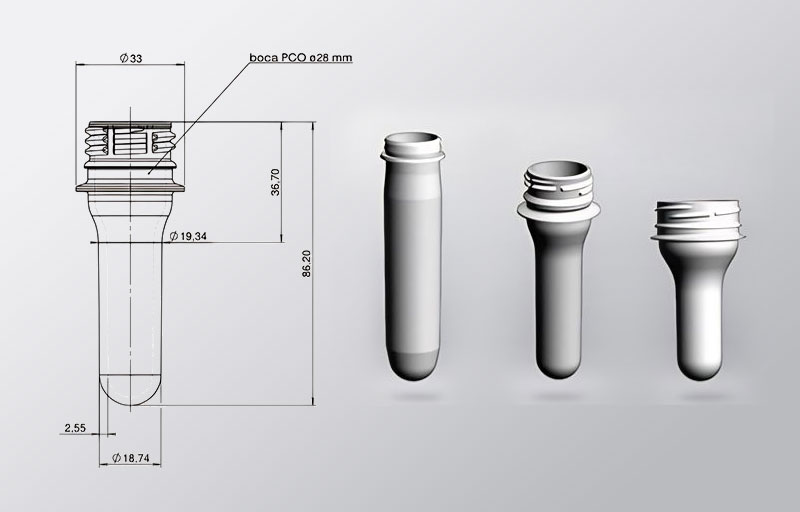 Cosmetic preform molds, while still requiring precision, allow for more flexibility in design. The focus is often on innovative shapes and ergonomic designs that enhance user experience. The tolerances may be slightly more lenient compared to medical molds, and there is greater scope for creativity in mold design.
Cosmetic preform molds, while still requiring precision, allow for more flexibility in design. The focus is often on innovative shapes and ergonomic designs that enhance user experience. The tolerances may be slightly more lenient compared to medical molds, and there is greater scope for creativity in mold design.
A significant difference lies in the surface finish requirements. Cosmetic preform molds emphasize a high-gloss finish and aesthetic detailing. Techniques like texturing, engraving, and embossing are commonly applied to enhance the visual appeal of cosmetic containers.
Medical preform molds prioritize functionality over aesthetics. The surface finish is typically smooth to prevent microbial adherence and facilitate sterilization. Any texturing is minimal and serves functional purposes, such as improving grip or facilitating product application.
The medical industry is heavily regulated. Medical preform molds must comply with standards such as ISO 10993 for biocompatibility and ISO 13485 for quality management systems. Manufacturers must ensure traceability of materials and comply with Good Manufacturing Practices (GMP).
Cosmetic preform molds are subject to less stringent regulations. While they must comply with safety standards and regulations like the FDA's guidelines for cosmetic products, the regulatory burden is lighter compared to medical devices. This allows for quicker product development cycles and more rapid innovation in design.
Medical preform molds must be capable of producing components that can withstand sterilization processes such as autoclaving, gamma irradiation, or ethylene oxide treatment. The materials and designs must not degrade or deform under these conditions.
For cosmetic preform molds, sterilization is not typically a requirement. However, cleanliness and hygiene are important to prevent contamination of cosmetic products. Standard cleaning procedures are usually sufficient to meet industry requirements.
Medical products are often produced in lower volumes compared to cosmetics. Consequently, medical preform molds may be designed for shorter production runs, with a focus on precision and reliability over speed. The cost per unit is generally higher due to the rigorous standards and lower economies of scale.
Cosmetic preform molds are designed for high-volume production. The molds must withstand continuous use and maintain consistency throughout long production runs. Economies of scale allow for a lower cost per unit, which is critical in the competitive cosmetic market.
The maintenance of medical preform molds requires strict adherence to protocols to prevent contamination. Regular validation and inspection are mandatory to comply with regulatory standards. The molds are often made from high-grade stainless steel to resist corrosion and facilitate sterilization.
Cosmetic preform molds also require maintenance to ensure product quality. However, the procedures are less stringent, focusing on preventing defects that could affect aesthetics. Materials used may vary, balancing cost with durability.
Advancements in technology have affected both medical and cosmetic preform molds. In the medical field, there is a growing use of automation and robotics to enhance precision and reduce human error. Technologies like cleanroom injection molding are becoming standard to meet cleanliness requirements.
In the cosmetic industry, innovations focus on rapid prototyping and flexible manufacturing systems. Technology enables quick changes in mold designs to adapt to market trends. Digital printing and other surface decoration technologies are also widely adopted.
Environmental concerns are influencing both sectors. Medical preform molds are beginning to incorporate sustainable materials where possible, although options are limited due to regulatory constraints. Efforts are focused on reducing waste and improving energy efficiency in manufacturing processes.
The cosmetic industry is proactively adopting sustainable practices. Biodegradable materials, recycled plastics, and eco-friendly packaging are becoming increasingly popular. Cosmetic preform molds are adapting to accommodate these new materials and designs.
Examining practical examples highlights the differences between medical and cosmetic preform molds. For instance, a manufacturer of medical syringes requires molds that produce components with micrometer precision and comply with FDA regulations. The production environment must be a certified cleanroom, and the molds undergo regular validation.
Conversely, a cosmetic company launching a new line of perfume bottles focuses on unique shapes and ornate designs. The preform molds must allow for intricate detailing and compatibility with decorative techniques. Production speed is crucial to meet market demand during promotional periods.
In the medical sector, quality control is rigorous. Each batch may require comprehensive testing, including dimensional analysis, material verification, and sterility checks. Documentation is critical for compliance and traceability.
Quality control in the cosmetic industry focuses on consistency and appearance. Visual inspections and sampling are common practices. While standards are high, the processes are generally less exhaustive than in medical manufacturing.
Understanding the differences between medical and cosmetic preform molds is essential for manufacturers and stakeholders in the plastics industry. Factors such as material selection, design precision, regulatory compliance, and production requirements vary significantly between the two sectors. By recognizing these differences, companies can better tailor their manufacturing processes to meet the specific needs of each industry.
